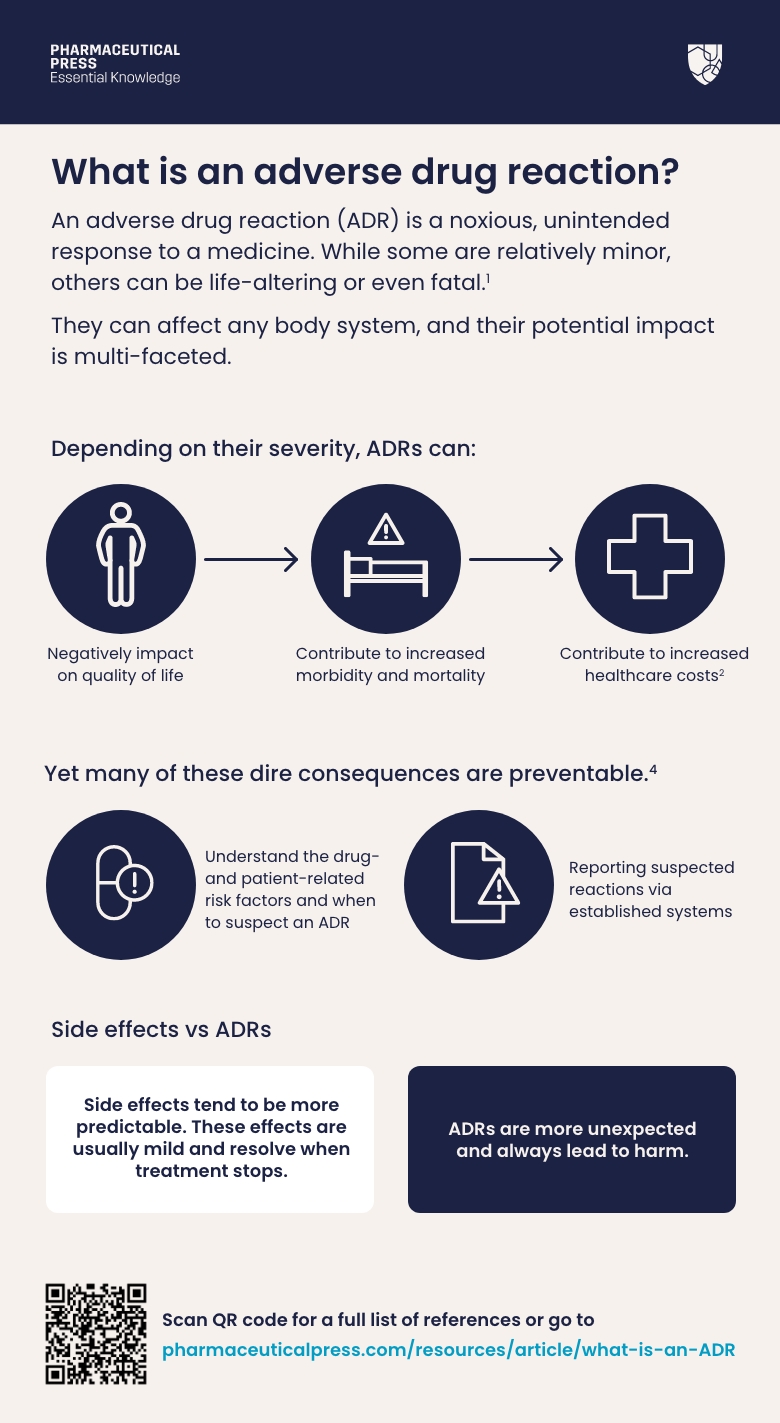
What is an adverse drug reaction?An adverse drug reaction (ADR) is a noxious, unintended response to a medicine. While some are relatively minor, others can be life-altering or even fatal.¹
An adverse drug reaction (ADR) is a noxious, unintended response to a medicine. While some are relatively minor, others can be life-altering or even fatal.¹
Please complete the form at the bottom of this article to request a complimentary trial of MedicinesComplete.
They can affect any body system, and their potential impact is multi-faceted. Depending on their severity, ADRs can negatively impact on quality of life, and contribute to increased morbidity, mortality, and healthcare costs.² By mimicking disease, they can also lead to unnecessary investigations, and result in delays to treatment.³
Yet many of these dire consequences are preventable.⁴ By understanding the drug- and patient-related risk factors and when to suspect an ADR, as well as reporting suspected reactions via established systems, health professionals can help to avoid the avoidable – and keep patients safe.
While the terms ADR and side effect are often used interchangeably, there are differences between the two.⁴ ⁵ Side effects tend to be more predictable; for example, many antibiotics can cause diarrhoea and sickness, but these effects are usually mild and resolve when treatment stops.
Some medicines can also be prescribed specifically for a known side effect. The drug minoxidil, initially used to treat high blood pressure, was noted to cause unwanted hair growth and is now widely used as a treatment for hair loss. ADRs, on the other hand, are more unexpected than side effects and always lead to harm.⁵
Types of adverse drug reactions
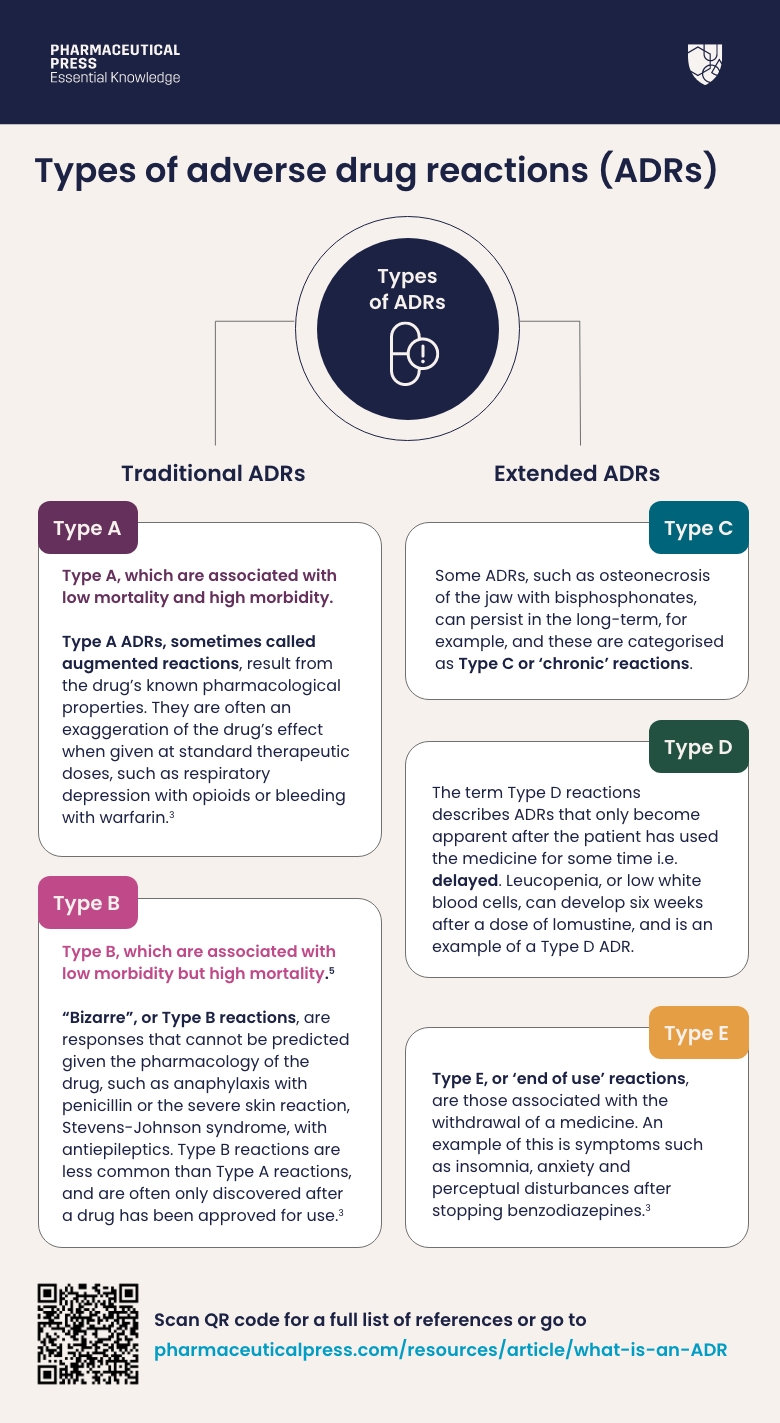
Traditionally, ADRs have been separated into two categories:²
Type A, which are associated with low mortality and high morbidity, and Type B, which are associated with low morbidity but high mortality.⁵
Type A ADRs, sometimes called augmented reactions, result from the drug’s known pharmacological properties. They are often an exaggeration of the drug’s effect when given at standard therapeutic doses, such as respiratory depression with opioids or bleeding with warfarin.³
“Bizarre”, or Type B reactions, are responses that cannot be predicted given the pharmacology of the drug, such as anaphylaxis with penicillin or the severe skin reaction, Stevens-Johnson syndrome, with antiepileptics. Type B reactions are less common than Type A reactions, and are often only discovered after a drug has been approved for use.³
However, this basic classification does not easily encompass all ADRs with varying clinical presentations. To account for this, the classification was extended to include a further three ADR categories. ³ ⁶
Some ADRs, such as osteonecrosis of the jaw with bisphosphonates, can persist in the long-term, for example, and these are categorised as Type C or ‘chronic’ reactions.
The term Type D reactions describes ADRs that only become apparent after the patient has used the medicine for some time i.e. delayed. Leucopenia, or low white blood cells, can develop six weeks after a dose of lomustine, and is an example of a Type D ADR.
Type E, or ‘end of use’ reactions, are those associated with the withdrawal of a medicine. An example of this is symptoms such as insomnia, anxiety and perceptual disturbances after stopping benzodiazepines.³
DoTS
A further classification system called ‘DoTS’ was subsequently proposed.² Dose-relatedness, time course, and susceptibility are components that can help to categorise ADRs:² As well as the dose (Do), the time course (T), of the reaction, and factors influencing the susceptibility of the patient (S), such as age and renal function, also need to be taken into account. This classification tool, although more comprehensive, is not widely used in clinical practice.
How common are adverse drug reactions?
Under-diagnosis and under-reporting has made estimating the prevalence of ADRs challenging.² We do know that they are common, but their frequency varies across healthcare settings and populations.
- In England, a 2021 systematic review and meta-analysis found that around 8% of people in primary care had experienced an ADR.⁷
- A 2004 prospective study of hospital admissions due to ADRs in Liverpool found a prevalence of 6.5% and estimated that around 2% of people admitted due to an ADR died.⁸
With information on ADRs for more than 1600 drugs used internationally, Martindale’s ADR Checker ensures quick decision-making at the point of care.
How common are preventable adverse drug reactions?
A similar recently published study found that an ADR was the primary or contributing cause of admission in 16.5% of hospital admissions over a 1 month period.⁹ It is concerning that this prevalence is a lot higher than that previously reported in the same hospitals and also that 40% of these ADRs were assessed as avoidable or possibly avoidable. Furthermore, 86.2% of these ADRs were Type A reactions,⁹ meaning they were due to recognised effects of the medicine(s) involved. Drug-drug interactions were also frequently implicated.
Understanding the prescribed medicine’s safety profile as well as factors influencing the individual patient’s susceptibility to adverse effects can help clinicians be alert to the possible risks.
Antiplatelets, anticoagulants, antineoplastics, immunosuppressants, diuretics, antidiabetics, and antibiotics are among the drug classes consistently implicated in hospital admissions. Fatal ADRs are relatively rare. A common cause is haemorrhage due to co-administration of an antithrombotic or anticoagulant with a non-steroidal anti-inflammatory drug (NSAID).⁴ A World Health Organization analysis of its pharmacovigilance database investigated reports of fatal ADRs in adults registered between 2010 and 2019. Of 23 million recorded ADRs, 43,685 were fatal. They were reported mainly in patients over 75 years old. The most frequently involved drug classes were antineoplastic/immunomodulating drugs followed by nervous system and cardiac drugs.¹⁰
Taking a thorough medication history, including any previous ADRs, can guide treatment decisions, enabling clinicians to avoid re-exposure. Regular medication review can minimise inappropriate polypharmacy. Age, ethnicity, pregnancy status, co-morbidities, renal and hepatic function and pharmacogenetic factors can also impact susceptibility, and should be considered carefully before prescribing.⁴
How much do adverse drug reactions cost?
ADRs have a high human cost. They can negatively impact on quality of life, and result in increased morbidity and mortality. ADRs often mimic common conditions, meaning people can be subjected to unnecessary tests and procedures, placing additional burden on patients and delaying time to effective treatment.² ³
They also carry significant financial implications, associated with hospital admissions, prolonged length of stay, and additional unnecessary interventions.⁹ ¹¹
As discussed, the most recent data suggest that preventable ADRs are responsible for around 16% of unplanned hospital admissions,⁹ a figure that may be even higher among older people.¹² Using these figures, the projected annual cost of ADR admissions to the NHS in England is £2.21bn.⁹ This does not take into the wider costs, such as ADRs occurring in the community.
How to manage adverse drug reactions
ADR management starts with accurate diagnosis. However, reactions can mimic almost any disease manifestation and this can make them challenging to detect.⁴ ¹³
Presentation can vary significantly, with any body system affected. Health professionals therefore need to consider the possibility of an adverse drug reaction in the differential diagnosis of a wide range of conditions.
Probably the most common reactions are those affecting the central nervous system (e.g. dizziness, headache or drowsiness), the gastrointestinal tract (e.g. nausea, vomiting), the skin (e.g. rashes), the liver (e.g. abnormal liver function tests) or the kidneys (e.g. acute kidney injury).
Rare ADRs, such as drug-induced lupus, fixed drug eruptions, and drug-induced eosinophilia or angioedema, are particularly challenging to detect.⁴ ¹⁴
It is essential to have a comprehensive medication history, including over-the-counter medicines and complementary therapies.
Asking the following questions can help health professionals identify connections between the symptom and the possibility of an ADR:⁴ ¹⁵
- Have you taken the medicine before without any issue? While previous treatment without problems doesn’t necessarily rule out an ADR, it may point against a drug cause.
- Did anything else change around the same time as the symptoms emerged? Were other treatments started, including over-the-counter medications, or is there anything to suggest disease progression? This can help pinpoint any alternative causes of the reaction.
- Did the reaction occur only after you started the drug? If symptoms developed before the patient started taking the drug, an ADR can usually be ruled out.
- Did the reaction get better when you stopped taking the drug? If the effect resolved after the treatment was stopped, the possibility of it being an ADR increases.
- Did you, either intentionally or accidentally, use the drug again after the reaction? If so, what happened? If the reaction happened again on re-exposure to the drug, the probability of a causal relationship increases.
Clinicans should also consider if additional investigations, such as liver function tests or gastrointestinal bleeding assessment, are required. In some cases, drug monitoring may be necessary. NICE recommends, for example, checking serum digoxin concentrations in people who have experienced suspected signs of toxicity.¹⁵
After diagnosis, treating the symptoms of an ADR accordingly, along with dose regimen alteration or drug withdrawal are the more common methods of management.⁴ ¹⁴
In some cases, an antidote or reversal treatment may be available. European Union legislation requires all new medicine approval submissions be accompanied by a robust risk management plan, which will sometimes involve the development of treatments for specify ADRs. Naloxone, for example, is widely used to treat opioid overdose, and idarucizumab restores dabigatran-prolonged coagulation parameters to baseline values.⁴
Adverse drug reactions and pharmacovigilance
Pharmacovigilance (PV) describes the process of monitoring the effects of medicines post-marketing approval in order to identify and evaluate any ADRs.¹⁶
While all new drugs go through extensive testing before being licensed for use, clinical trials tend to involve a few thousand carefully selected patients at most. Rare ADRs, however, may not become apparent until hundreds of thousands of people have used the medicine.
What’s more, the strict conditions governing medicines use in clinical trials do not always reflect how they will be used in real life. The drugs may be taken, for example, by millions of people across a wide range of age groups who may also be taking other medicines, and who have varied lifestyles and/or complex co-morbidities.
Post-marketing drug safety surveillance (PMS) aims to identify safety signals, or information on a new or known ADR that may be caused by a drug and requires further investigation, in larger populations. It is essential for protecting the safety of individual patients, and provides health professionals with a better understanding of a drug’s safety profile, thereby guiding informed prescribing.¹⁷
PMS involves the continuous monitoring of medicines once they reach the market to detect, assess, and understand ADRs and side effects so that, where possible, they can be prevented. It starts the moment that a drug is licensed and continues for the entire period that the medicine is available for patient use.
Reporting adverse drug reactions
In the UK, the MHRA monitors the use of medicines in everyday practice using the Yellow Card Scheme.³ While reporting is voluntary, the Royal Pharmaceutical Society recommends that pharmacists report all serious suspected ADRs they encounter as a matter of best practice, even if the effect is well recognised.¹⁸
Reports can be made for reactions relating to vaccines, blood factors and immunoglobins, complementary medicines, medical devices, or defective or low quality medicines, as well as falsified medicines or devices and e-cigarette products.²
Products marked with an inverted black triangle on the patient and prescribing information are under EU-wide additional monitoring.¹⁹ The black triangle scheme covers drugs being closely monitored by regulatory bodies, as there is often relatively little safety information from clinical trials. The scheme covers newly marketed medicines, those with new routes of administration or new delivery systems, and established medicines being used in a new patient population.³
Any suspected ADR that occurs with a black triangle medicine should be reported using the Yellow Card Scheme. The MHRA uses case report and other data to assess the status of Black Triangle products after they have been on the market for two years, and the symbol is not removed until the drug’s safety is well established.²⁰ ²¹
Avoiding the avoidable
In conclusion, ADRs are common, and are a critical threat to patient safety and healthcare costs.²²
An understanding of how to classify reactions into types, and how to explore whether a medicine is the potential cause of an adverse effect could help clinicians better identify them.
Preventing ADRs through careful prescribing practices, avoiding polypharmacy, monitoring and reviewing medication use regularly, and, more often in current practice, being aware of pharmacogenetic factors, remains vital in minimising harm to patients. The greatest potential for harm is in people with multimorbidity, such as multiple long-term conditions affecting different organ systems. Other factors are siloed treatment strategies, e.g. across primary and secondary care, and sometimes conflicting and interacting treatment regimes. In those people at greatest risk, regular scrutiny of all prescribed medicines is essential to prevent ADRs and interactions. A multidisciplinary approach, spanning primary and secondary care practitioners, is key to successful, sustainable medicines optimisation.²² Accurate diagnosis and prompt reporting of appropriate reactions via the Yellow Card Scheme are essential steps in safeguarding public health from preventable harm.
Trial form
Please complete the form below to request a complimentary trial to knowledge products through MedicinesComplete.
References
1. EMA. (n.d.). Adverse drug reaction. Available at: https://www.ema.europa.eu/en/glossary-terms/adverse-drug-reaction Last accessed: 30 September 2024
2. Jutley, G. S., Pucci, M., et al. (2023). Adverse drug reactions and interactions. Medicine. 52(1),15-22.
3. MHRA. (n.d.) Guidance on adverse drug reactions. Available at: https://assets.publishing.service.gov.uk/media/5feefb4c8fa8f53b7a0fbe36/Gudance_on_adverse_drug_reactions.pdf Last accessed: 30 September 2024.
4. Coleman, J. J., & Pontefract, S. K. (2016). Adverse drug reactions. Clinical Medicine. 16(5), 481-485.
5. IU Phar. (n.d.). Adverse drug reactions. Available at: https://www.pharmacologyeducation.org/clinical-pharmacology/adverse-drug-reactions Last accessed: 30 September 2024
6. Kaufman, G. (2016). Adverse drug reactions: classification, susceptibility and reporting. Nursing Standard, 30(50).
7. Insani, W. N., Whittlesea, C., et al (2021). Prevalence of adverse drug reactions in the primary care setting: A systematic review and meta-analysis. PLoS One, 16(5), e0252161.
8. Pirmohamed, M., James, S., et al. (2004). Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. Bmj, 329(7456), 15-19.
9. Osanlou, R., Walker, L., et al. (2022). Adverse drug reactions, multimorbidity and polypharmacy: a prospective analysis of 1 month of medical admissions. BMJ open, 12(7), e055551.
10. Montastruc JL, Lafaurie M, de Canecaude C, Durrieu G, Sommet A, Montastruc F & Bagheri H (2021). Fatal adverse drug reactions: a worldwide perspective in the world health organization pharmacovigilance database.. British Journal of Clinical Pharmacology, 87(11), 4334-4340. https://dx.doi.org/10.1111/bcp.14851
11. Formica, D., Sultana, J., et al. (2018). The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert opinion on drug safety, 17(7), 681-695.
12. Kongkaew, C., Noyce, P. R., et al. (2008). Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Annals of Pharmacotherapy, 42(7-8), 1017-1025.
13. NICE. (2015) Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. Available at: https://www.nice.org.uk/guidance/ng5/resources/medicines-optimisation-the-safe-and-effective-use-of-medicines-to-enable-the-best-possible-outcomes-pdf-51041805253 Last accessed: 30 September 2024.
14. NICE. (2022). Adverse drug reactions. Available at: https://cks.nice.org.uk/topics/adverse-drug-reactions/ Last accessed: 30 September 2024.
15. NICE. (2022). Adverse drug reactions :Scenario: Adverse drug reactions. Available at: https://cks.nice.org.uk/topics/adverse-drug-reactions/management/adverse-drug-reactions/ Last accessed: 30 September 2024
16. WHO. (n.d). Pharmacovigilance. Available at: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance Last accessed: 30 September 2024
17. Alomar, M., Tawfiq, A. M., et al. (2020). Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Therapeutic advances in drug safety, 11, 2042098620938595.
18. RPS. (n.d.) Reporting adverse events. Available at: https://www.rpharms.com/mep/2-core-concepts-and-skills/25-patient-safety/253-reporting-adverse-events#gsc.tab=0 Last accessed: 30 September 2024.
19. MHRA. (n.d.) Types of Yellow Card reports. Available at: https://yellowcard.mhra.gov.uk/resources/reporttypes Last accessed: 30 September 2024.
20. EMA. (n.d.) Medicines under additional monitoring. Available at: https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/medicines-under-additional-monitoring Last accessed: 30 September 2024
21. UK Government. (2014). The Black Triangle scheme. Available at: https://www.gov.uk/drug-safety-update/the-black-triangle-scheme-or Last accessed: 30 September 2024.
22. Walker LE & Pirmohamed M (2023). Increasing trend in hospitalisation due to adverse drug reactions: can we stem the tide?. Drug & Therapeutics Bulletin, 61(6), 87-91. https://dx.doi.org/10.1136/dtb.2022.000050