What is a drug interaction?The definition of a drug-drug interaction
A drug interaction is said to occur when the effects of one drug are changed by the presence of another drug, herbal medicine, food, drink, or by some environmental chemical agent.
The outcome can be harmful if the interaction causes an increase in the toxicity of the drug. For example, there can be an increase in risk of severe muscle damage if patients taking statins start taking azole antifungals, and patients taking monoamine oxidase inhibitor antidepressants (MAOIs) might experience an acute and potentially life-threatening hypertensive crisis if they eat tyramine-rich foods such as cheese.
A reduction in efficacy due to an interaction can sometimes be just as harmful as an increase: patients taking warfarin who are given rifampicin (rifampin) need more warfarin to maintain adequate anticoagulation, while patients taking tetracyclines or quinolones need to avoid antacids and milky foods (or separate their ingestion) because the effects of these antibacterials can be reduced or even abolished if admixture occurs in the gut.
These unwanted and unsought interactions are adverse and undesirable but there are other interactions that can be beneficial and valuable, such as the deliberate coprescription of antihypertensive drugs and diuretics in order to achieve antihypertensive effects possibly not obtainable with either drug alone.
Please complete the form at the bottom of this article to request a complimentary trial of MedicinesComplete.

What is a drug interaction?
Access the Stockley’s Interactions Checker, a quick and easy-to-use drug interaction checking tool, and the full-text reference publication, Stockley’s Drug Interactions, online through MedicinesComplete.
Search for drug interactions using Stockley’s Interactions Checker.
What is the incidence of drug interactions?
The more drugs a patient takes the greater the likelihood that an adverse reaction will occur. One hospital study found that the rate was 7% in those taking 6 to 10 drugs but 40% in those taking 16 to 20 drugs, which represents a disproportionate increase.¹ A possible explanation is that the drugs were interacting.
Some of the early studies on the frequency of interactions uncritically compared the drugs that had been prescribed with lists of possible drug interactions, without appreciating that many interactions can be clinically trivial or simply theoretical. As a result, an unrealistically high incidence was suggested. Most of the later studies have avoided this error by looking at only potentially clinically important interactions, and incidences of up to 8.8% have been reported.²⁻⁴ Even so, not all of these studies took into account the distinction that must be made between the incidence of potential interactions and the incidence of those where clinical problems actually arise. The simple fact is that some patients experience quite serious reactions while taking interacting drugs, while others appear not to be affected at all.
The discordant figures regarding the incidence of drug interactions that are reported in studies need to be put into the context of the under-reporting of adverse reactions by health professionals, for reasons that might include pressure of work or the fear of litigation. Both health professionals and patients might not recognise adverse reactions and interactions, and some patients simply stop taking their drugs without saying why. No study gives a clear answer to the question of how frequently drug interactions occur, but even if the incidence is as low as some studies suggest, it still represents a very considerable number of patients who appear to be at risk when one thinks of the large numbers of drugs prescribed and taken every day.

What is a drug interaction? Drug-food interactions accessed through Stockley’s Interactions Checker.
What are some examples of food and drug interactions?
It is well established that food can cause clinically important changes in drug absorption through effects on gastrointestinal motility or by drug binding; for example, a high-fat meal decreases the absorption of afatinib, resulting in a decrease in its exposure. In addition, it is well known that tyramine (present in some foodstuffs) might reach toxic concentrations in patients taking MAOIs and can cause a potentially life-threatening hypertensive reaction.
With the growth in understanding of drug metabolism mechanisms, it has been increasingly recognised that some foods can alter drug metabolism. For example, cruciferous vegetables, such as brussels sprouts, cabbage, and broccoli, contain substances that are inducers of the cytochrome P450 isoenzyme, CYP1A2, which is involved in the metabolism of several drugs. Chemicals formed by ‘burning’ meats additionally have these properties. These foods do not appear to cause any clinically important drug interactions in their own right, but their consumption can add another variable to drug interaction studies, so complicating interpretation.
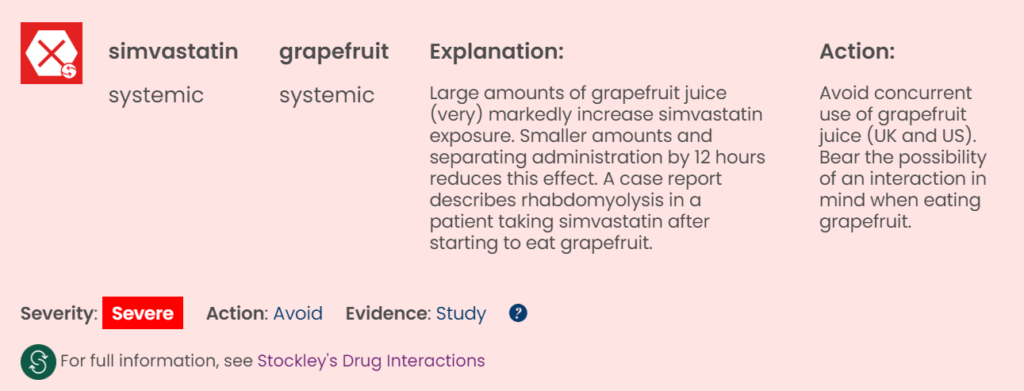
Drug-grapefruit interactions accessed through Stockley’s Interactions Checker.
Drug-grapefruit interactions
In 1989 a group of researchers investigating the influence of ethanol on the haemodynamic effects of felodipine discovered that when felodipine was administered with grapefruit juice (used to mask the flavour of the alcohol), felodipine plasma concentrations were 7-fold higher than expected.⁵.
Since then, grapefruit juice has been extensively studied. It is now known to weakly inhibit CYP3A4, and that the predominant mechanism responsible for the increased exposure to felodipine is specifically the inhibition of CYP3A4 in the small intestine. Evidence is also emerging that shows that other fruit juices (such as bitter (Seville) orange) might affect the exposure to a variety of drugs by inhibiting CYP3A4.
Fruit and fruit juices are known to have several potentially pharmacologically active compounds, including flavonoids (such as naringin and hesperidin) and furanocoumarins (such as bergamottin and dihydroxybergamottin). It is not certain which components of fruit juice are responsible for interacting with drugs. Naringin, which is metabolised during processing to naringenin, is a substance known to inhibit CYP3A4, and it has been suggested that bergamottin, and dihydroxybergamottin are also responsible for CYP3A4 inhibition. These components, which are all found in grapefruit juice, inhibit the activity of CYP3A4 in the intestine, which leads to an increase in the exposure and effects of drugs that are metabolised by CYP3A4.
The product literature for drugs that are metabolised by CYP3A4 often warns against the concurrent use of grapefruit juice and/or the fruit itself. Although grapefruit juice is generally considered to be a weak CYP3A4 inhibitor, its effects can be variable.
There is known to be some variability in the components of citrus juice products, which can be a result of both natural and commercial factors. The concentration of natural compounds within each fruit varies with fruit variety, climate, and environmental conditions during fruit development and fruit maturity. In addition, the profile of compounds in citrus juice can be influenced during commercial juicing procedures. For example, the mechanical pressing of fruit increases contact between areas of the fruit such as the peel and the pith which have a much higher concentration of naringin than the juice vesicles. The compounds can also be influenced by the process of debiting or turning the juice into a concentrate.⁶ Therefore the components responsible for interactions appear in different quantities in different products of the same type of juice, making interactions between drugs and fruit juices difficult to manage.
When it comes to the question of juice versus whole fruit, some research has been carried out with grapefruit. Various studies have shown that grapefruit pulp, grapefruit segments, or grapefruit segment-free extract can increase the AUC of nifedipine, nisoldipine, and felodipine by 30%, 30%, and 3-fold, respectively.⁷⁻⁸ However, a case study found that ingestion of a grapefruit (300 g) before taking either amlodipine or nifedipine had no effect on the plasma concentration of either drug.⁹
Some drugs that are not metabolised by CYP3A4, such as fexofenadine, show decreased exposure on concurrent use with grapefruit juice. The probable reason for this is that grapefruit juice is also an inhibitor of drug transporters, such as P-glycoprotein and organic anion-transporting polypeptide (OATP). Other fruit juices can also affect these transporters.
References
- Smith JW, Seidl LG, Cluff LE. Studies on the epidemiology of adverse drug reactions. V. Clinical factors influencing susceptibility. Ann Intern Med (1969) 65, 629.
- Puckett WH, Visconti JA. An epidemiological study of the clinical significance of drug-drug interaction in a private community hospital. Am J Hosp Pharm (1971) 28, 247.
- Shinn AF, Shrewsbury RP, Anderson KW. Development of a computerized drug interaction database (Medicom) for use in a patient specific environment. Drug Inf J (1983) 17, 205.
- Ishikura C, Ishizuka H. Evaluation of a computerized drug interaction checking system. Int J Biomed Comput (1983) 14, 311.
- Bailey DG, Spence JD, Edgar B, Bayliff CD, Arnold JM. Ethanol enhances the hemodynamic effects of felodipine. Clin Invest Med (1989) 12(6) 357-362.
- Ameer B, Weintraub R.A, Drug Interactions with Grapefruit Juice. Clin. Pharmacokinetics(1997) (2) 103-121.
- Ohtani M, Kawabata S, Kariya S, Uchino K, Itou K, Kotaki H, Kasuyama K, Morikawa A, Seo I, Nishida N. Effect of grapefruit pulp on the pharmacokinetics of the dihydropyridine calcium antagonists nifedipine and nisoldipine. Yakugaku Zasshi (2002) 122, 323–9.
- Bailey DG, Dresser GK, Kreeft JH, Munoz C, Freeman DJ, Bend JR. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther (2000) 68, 468–77.
- Nakagawa K, Goto T. Effects of ingestion of grapefruit juice or grapefruit on the hypotensive effect and plasma concentrations of dihydropyridine calcium antagonists (amlodipine and nifedipine): a case study. Clin Exp Hypertens (2010) 32, 71–5.