Pharmacokinetic and pharmacodynamic relationshipsWhat is pharmacodynamics?
It has been said that pharmacokinetics describes what the body does to the drug (absorption, distribution and elimination); while pharmacodynamics measures what the drug does to the body (therapeutic and/or toxic effect).
![Sigmoidal relationship between effect (forced expiratory volume in 1 s [FEV1]) and plasma drug concentration for albuterol.](data:image/svg+xml;base64,PHN2ZyB4bWxucz0iaHR0cDovL3d3dy53My5vcmcvMjAwMC9zdmciIHdpZHRoPSI3ODAiIGhlaWdodD0iNjAwIiB2aWV3Qm94PSIwIDAgNzgwIDYwMCI+PHJlY3Qgd2lkdGg9IjEwMCUiIGhlaWdodD0iMTAwJSIgc3R5bGU9ImZpbGw6I2NmZDRkYjtmaWxsLW9wYWNpdHk6IDAuMTsiLz48L3N2Zz4=)
Figure 17.1: Sigmoidal relationship between effect (forced expiratory volume in 1 s [FEV1]) and plasma drug concentration for albuterol.
Pharmacokinetics and pharmacodynamics
The entire science of pharmacokinetics is predicated on the observation that, for most drugs, there is a correlation between drug response and drug concentration in the plasma. This correlation is not, however, a linear one. In fact, for most drugs, a sigmoidal (S-shaped) relationship exists between these two factors.
What is pharmacodynamics?
It has been said that pharmacokinetics describes what the body does to the drug (absorption, distribution and elimination); while pharmacodynamics measures what the drug does to the body (therapeutic and/or toxic effect).
![Sigmoidal relationship between effect (forced expiratory volume in 1 s [FEV1]) and plasma drug concentration for albuterol.](https://www.pharmaceuticalpress.com/wp-content/uploads/2025/01/pharmacokinetics-and-pharmacodynamics-graph-1.jpg)
Figure 17.1: Sigmoidal relationship between effect (forced expiratory volume in 1 s [FEV1]) and plasma drug concentration for albuterol.
The entire science of pharmacokinetics is predicated on the observation that, for most drugs, there is a correlation between drug response and drug concentration in the plasma. This correlation is not, however, a linear one. In fact, for most drugs, a sigmoidal (S-shaped) relationship exists between these two factors.
In Figure 17.1, the therapeutic effect reaches a plateau, where increase in drug concentration will have no further increase in effect. In contrast, the toxic effects of a drug show no such plateau.
Toxic effects start at the minimum toxic concentration and continue to rise, without limit, as drug concentration increases (Figure 17.2).
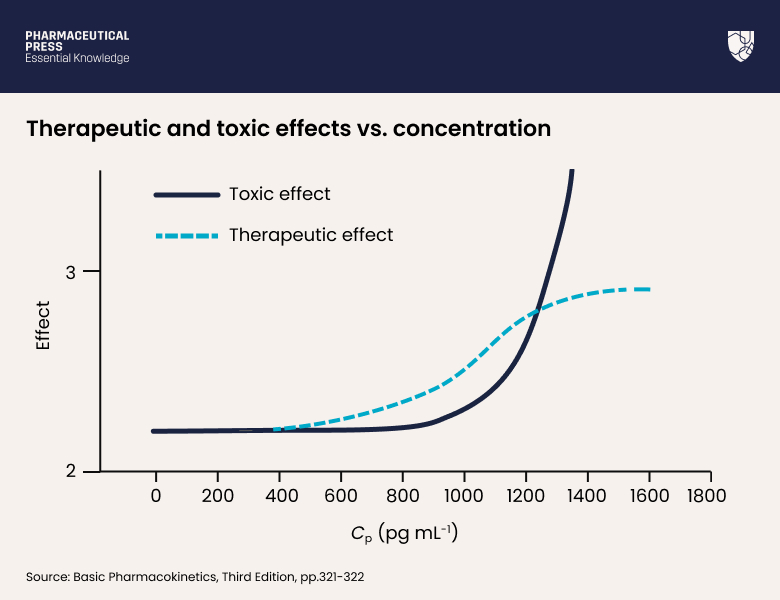
Figure 17.2: While therapeutic effects reach a plateau, toxic effects continue to rise with increasing drug concentration (Cp). Albuterol therapeutic effect is measured by the forced expiratory volume in 1 s (FEV1); while its toxic effects are mainly cardiovascular.
Upon consideration, it is clear that the best measure of a drug’s activity at any given time would be obtained from a direct and quantitative measurement of the drug’s therapeutic effect. This is, in fact, possible for a few drugs. For example, the effect of an antihypertensive drug is best measured by recording the patient’s blood pressure. There is no need to determine plasma drug concentrations of these drugs. However, for the large majority of drugs whose effect is not quantifiable, the plasma drug concentration remains the best marker of effect.
The content of this article has been taken from Basic Pharmacokinetics Third Edition. Order here.
The science of pharmacokinetics allows us to determine a drug dose and dosing interval to achieve and maintain a plasma drug concentration within the therapeutic range. We can also predict the time course of plasma drug concentration over time, observing fluctuations and deciding when a declining concentration becomes low enough to require the administration of another dose.
PKPD model
For some drugs, we can link the parameters and equations of pharmacokinetics to those of pharmacodynamics, resulting in a PKPD model which can predict pharmacological effect over time. This concept is discussed in more detail in Basic Pharmacokinetics Third Edition. (Equation 7 is a typical PKPD equation.) Figure 17.3 depicts the relationship of effect versus time for the drug albuterol (salbutamol) and contrasts this with a superimposed plot of plasma drug concentration versus time.
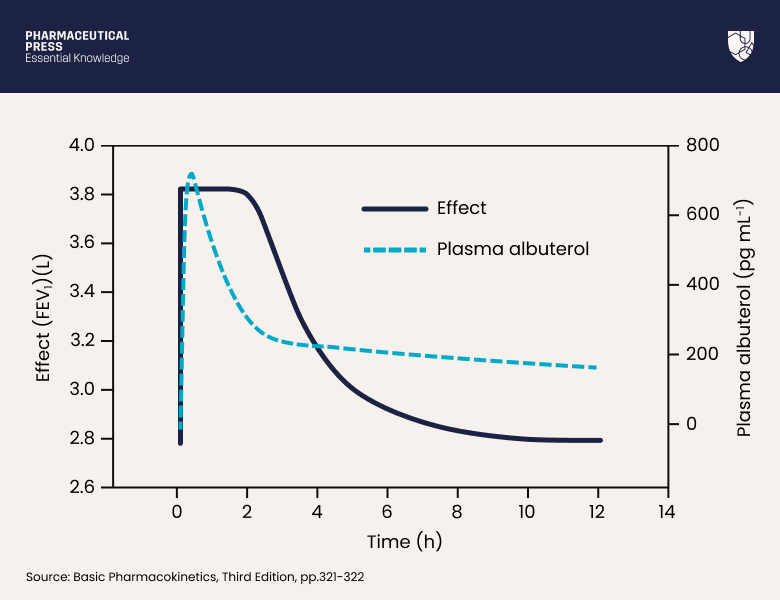
Figure 17.3: Drug effect (solid curve) versus time contrasted with drug concentration (dashed curve) versus time. Albuterol effect is measured by the forced expiratory volume in 1 s (FEV1).
Written by the authors of Basic Pharmacokinetics Third Edition Sunil S. Jambhekar and Philip J. Breen
For more pharmacokinetics and pharmacodynamics information, try our complimentary trial of MedicinesComplete for health professionals.












