The MHRA Green Guide
The MHRA Green Guide
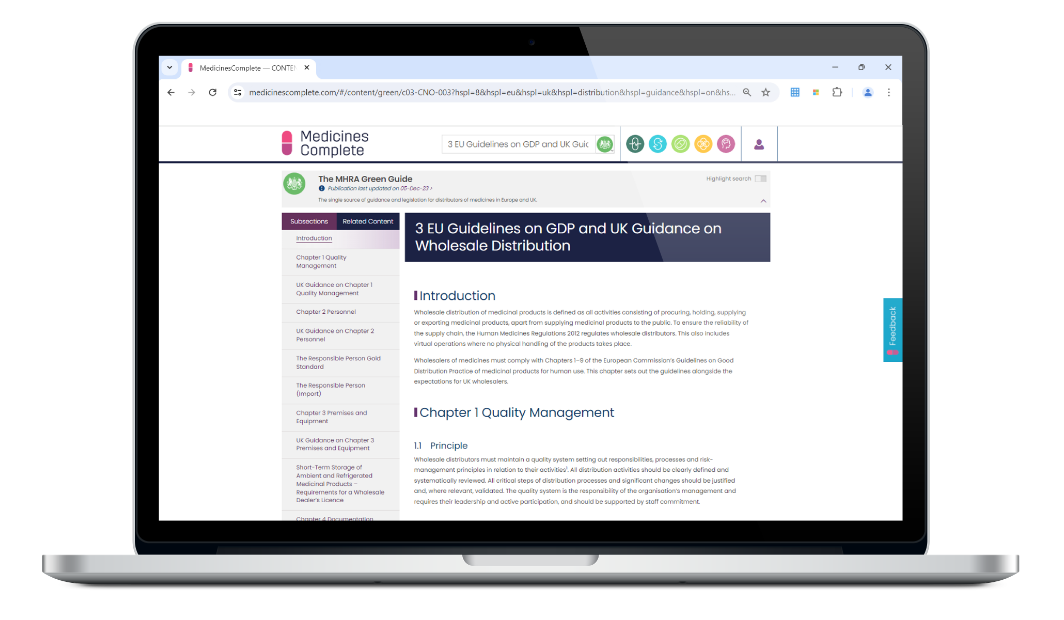

The reference source for distributors of medicines
The MHRA Green Guide is the essential reference for all distributors, brokers of human medicines, importers, and distributors of active substances in Europe and the UK.
Rules and Guidance for Pharmaceutical Distributors (known as The MHRA Green Guide) is compiled by the Medicines and Healthcare Regulatory Agency (MHRA) as the single authoritative source of European good distribution practices and UK guidance, information and UK legislation on these activities.
- 5,000+
- Page views
- 50+
- Users per month
- 160+
- Searches
Supports medicines distributors in meeting legal requirements

GDP guidelines for active substances

UK guidance and legislation on brokering medicines

Guidance on pharmacovigilance for wholesalers

Minimises research time

Aids good practice

Incorporates changes made after the UK’s exit from the European Union

Naming of sites on a licence

Provides a framework for continuous improvement in quality control and monitoring
Request access today
Access The MHRA Green Guide through MedicinesComplete today.
Contact us now for pricing and access information.
Related resources
Related publications
-
 BEST SELLER
BEST SELLERRules and Guidance for Pharmaceutical Manufacturers and Distributors (The MHRA Orange Guide) 2022
The reference for manufacturers and distributors of medicines in Europe and the UK
Info -
 NEW
NEWBritish National Formulary (BNF) 90
The first choice for concise medicines informationPractical and evidence based, British National…
Info -
 NEW
NEWMedicines, Ethics and Practice Edition 48
The Royal Pharmaceutical Society’s established professional guide for pharmacists
Info
See all our printed publications in the Shop.