10th February 2026
British National Formulary
This update contains 12 significant changes, 3 dose changes and…


Psychotropic Drug Directory supports the optimal and rational use of medicines, to improve the quality of life for people with mental health needs.
Close
Close

Contact our team to see how our services can directly help you.
Close

For new and experienced users, get the most out of your access to MedicinesComplete with our handy tips and shortcuts. Save time and get to the information you need quickly.
Close

Register for this webinar to learn how MedicinesComplete can support you in implementing effective processes to reduce medication errors and improve patient safety.
Close

Here’s a handy reminder of all the patient safety topics we’ve covered.
Close
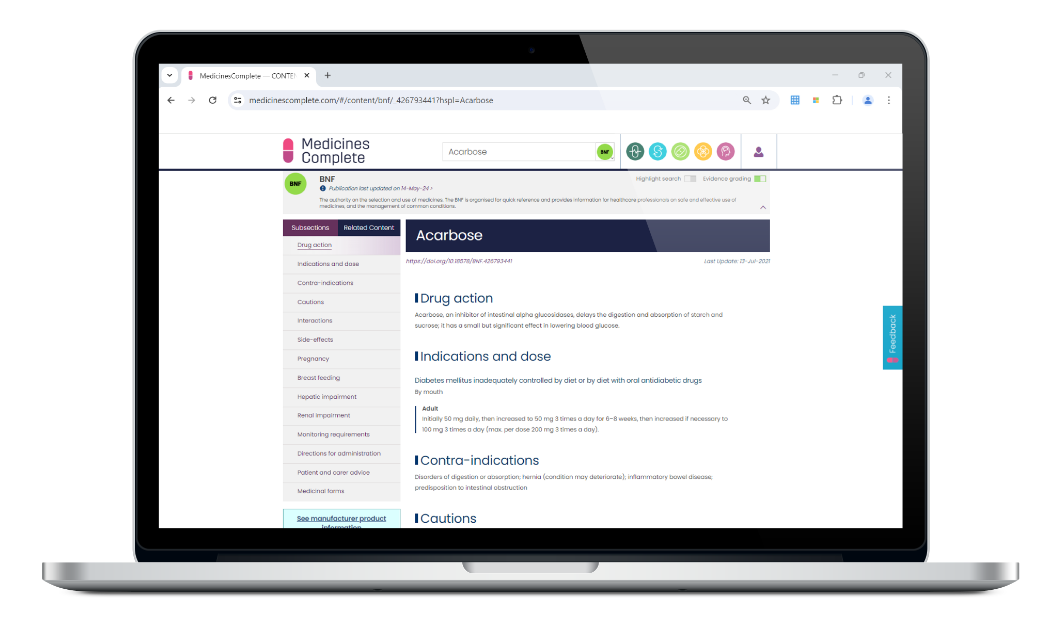

Practical and evidence based, British National Formulary (BNF) is the only drug formulary in the world that is both independent, and has rigorous, accredited content creation processes.
An integral part of the UK’s healthcare infrastructure and relied on by health professionals who prescribe, dispense, and administer medicines globally. Reflecting current best practice as well as legal and professional guidelines relating to the uses of medicines, BNF supports safe and effective decision-making at the point of care.






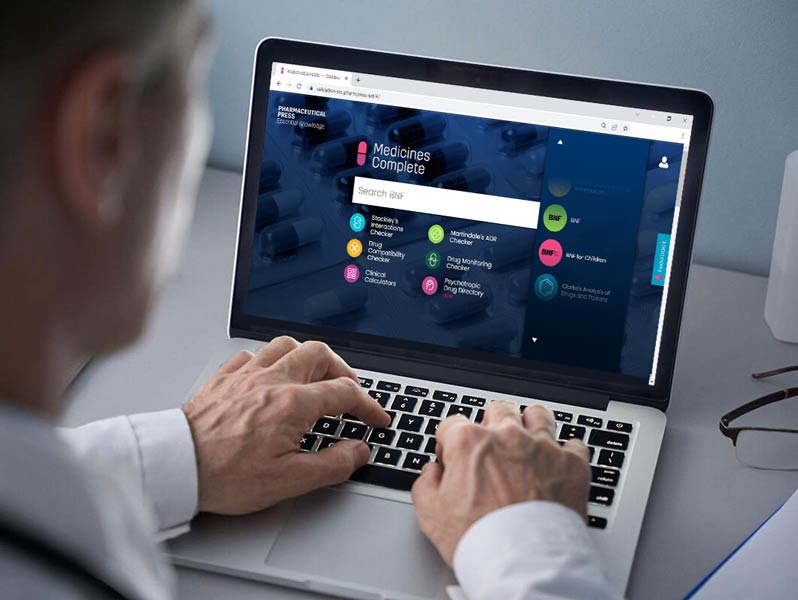
Our knowledge products are regularly updated online through MedicinesComplete
10th February 2026
This update contains 12 significant changes, 3 dose changes and…
10th February 2026
This update contains 12 significant changes, 3 dose changes and 2 new monographs.
Significant Changes:
Dose Changes:
New Monographs:
13th January 2026
This update contains 15 significant changes, 3 dose changes, 4…
13th January 2026
This update contains 15 significant changes, 3 dose changes, 4 new
monographs, 1 deleted monograph, and 1 deleted preparation.
Significant Changes:
Dose Changes:
New Monographs:
Deleted Monographs: Varicella-zoster immunoglobulin
Deleted Preparations: Varicella-zoster immunoglobulin solution for injection (Bio Products Laboratory).
9th December 2025
This update contains 15 significant changes,1 dose change, 2 new…
9th December 2025
This update contains 15 significant changes,1 dose change, 2 new preparations
and 11 deleted monographs.
Significant Changes:
Dose Changes:
New Preparations: Enalto® [escitalopram]; Rybelsus® 1.5 mg, 4 mg, and 9 mg round tablets [semaglutide].
Deleted Monographs: Adefovir dipivoxil; Aspirin with codeine; Bezlotoxumab;
Castor oil with collodion and colophony; Colestipol hydrochloride; Diclofenac;
Macrogol 3350 with anhydrous sodium sulfate, potassium chloride, sodium.
11th November 2025
This update contains 32 significant changes, 3 dose changes, 3…
11th November 2025
This update contains 32 significant changes, 3 dose changes, 3 new monographs, 1 new preparation, and 1 deleted preparation.
Significant Changes:
Dose Changes:
New Monographs:
New Preparations: Paclitaxel albumin-bound
Deleted Preparations: Victoza® [liraglutide].
14th October 2025
This update contains 10 significant changes, 3 new monographs and…
14th October 2025
This update contains 10 significant changes, 3 new monographs and 1 deleted monograph
Significant Changes:
New Monographs:
Deleted Monographs: Hepatitis A with typhoid vaccine.
9th September 2025
This update contains 8 significant changes, 1 dose change, 3…
9th September 2025
This update contains 8 significant changes, 1 dose change, 3 new monographs and 1 new preparation.
Significant Changes:
Dose Changes:
New Monographs:
New Preparations: Tysabri® solution for injection in pre-filled syringe [natalizumab].
Access BNF through MedicinesComplete today.
Contact us now for pricing and access information.
BNF and BNFC are published jointly by BMJ Group, Pharmaceutical Press, RCPCH, and NPPG.
A significant investment from National Institute for Health and Care Excellence (NICE) ensures that, with our partners BMJ Group, RCPCH, and NPPG, we support NHS health professionals in the UK with unlimited access to BNF and BNFC content online through MedicinesComplete, via the BNF + BNFC app.
The Uniform Requirements for Manuscripts Submitted to Biomedical Journals (http://www.icmje.org), formerly referred to as the Vancouver convention, is preferred by many medical journals.
The style for referencing the printed version of BNF using the Uniform Requirements for Manuscripts is as follows:
Joint Formulary Committee. British National Formulary. [edition number] ed. London: BMJ and Pharmaceutical Press; [year of publication]
The style for referencing the online version of BNF is as follows:
Joint Formulary Committee. British National Formulary (online) London: BMJ and Pharmaceutical Press <http://www.medicinescomplete.com> [Accessed on [date]]
The style for referencing the printed version of BNF for Children using the Uniform Requirements for Manuscripts is as follows:
Paediatric Formulary Committee. BNF for Children [edition year]. London: BMJ, Pharmaceutical Press, and RCPCH Publications; [year of publication]
The style for referencing the online version of BNF for Children is as follows:
Paediatric Formulary Committee. BNF for Children (online) London: BMJ, Pharmaceutical Press, and RCPCH Publications <http://www.medicinescomplete.com> [Accessed on [date]]

Info

Info

Info
See all our printed publications in the Shop.