10th February 2026
BNF for Children
This update contains 9 significant changes, 3 dose changes and…


Psychotropic Drug Directory supports the optimal and rational use of medicines, to improve the quality of life for people with mental health needs.
Close
Close

Contact our team to see how our services can directly help you.
Close

For new and experienced users, get the most out of your access to MedicinesComplete with our handy tips and shortcuts. Save time and get to the information you need quickly.
Close

Register for this webinar to learn how MedicinesComplete can support you in implementing effective processes to reduce medication errors and improve patient safety.
Close

Here’s a handy reminder of all the patient safety topics we’ve covered.
Close
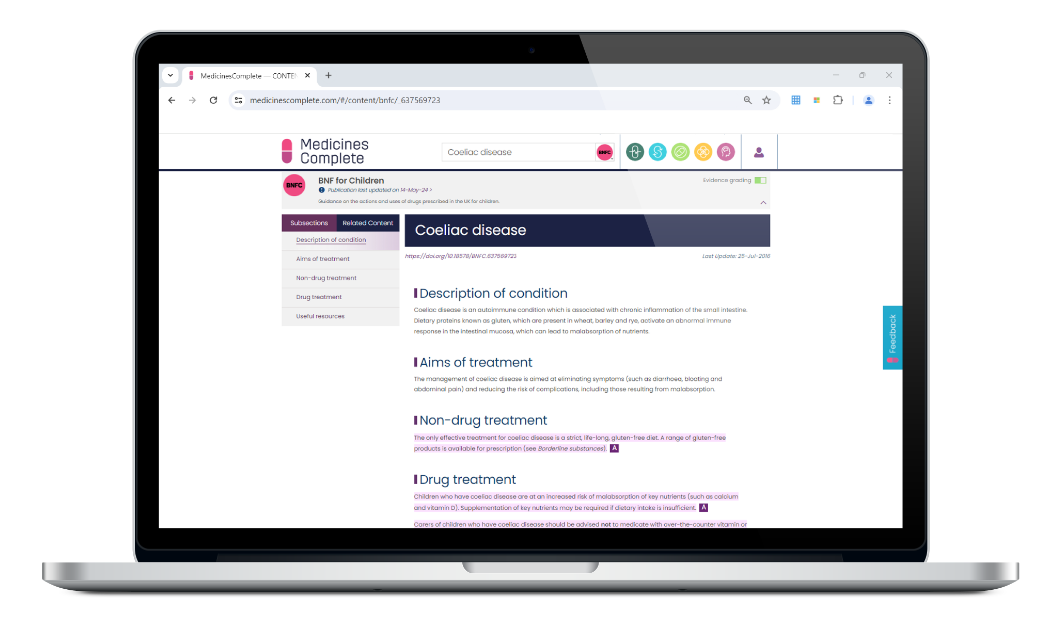

Practical and evidence-based, BNFC is the only child-focused drug formulary in the world that is both independent, and has rigorous, accredited content creation processes.
Relied on by health professionals who prescribe, dispense, and administer medicines for childhood disorders, BNFC supports confident decision-making at the point of care. Including best practice guidelines and advice from a network of paediatric experts. The process is overseen by a Paediatric Formulary Committee.






Our knowledge products are regularly updated online through MedicinesComplete
10th February 2026
This update contains 9 significant changes, 3 dose changes and…
10th February 2026
This update contains 9 significant changes, 3 dose changes and 1 new monograph.
Significant Changes:
Dose Changes:
New Monographs:
13th January 2026
This update contains 15 significant changes, 4 dose changes, 1…
13th January 2026
This update contains 15 significant changes, 4 dose changes, 1 new monograph,
1 new preparation, and 1 deleted preparation.
Significant Changes:
Dose Changes:
New Monographs:
New Preparations: Varitect CP® [varicella-zoster immunoglobulin]
Deleted Preparations: Varicella-zoster immunoglobulin solution for injection (Bio Products Laboratory).
9th December 2025
This update contains 9 significant changes, and 5 deleted monographs….
9th December 2025
This update contains 9 significant changes, and 5 deleted monographs.
Significant Changes:
Deleted Monographs: Castor oil with collodion and colophony; Colestipol hydrochloride; Macrogol 3350 with anhydrous sodium sulfate, potassium chloride, sodium bicarbonate and sodium chloride; Potassium bicarbonate with potassium acid tartrate; Ranitidine.
11th November 2025
This update contains 31 significant changes, 2 new monographs, and…
11th November 2025
This update contains 31 significant changes, 2 new monographs, and 1 deleted monograph
Significant Changes:
New Monographs:
Deleted Monographs: Victoza® [liraglutide].
14th October 2025
This update contains 7 significant changes, 5 new monographs and…
14th October 2025
This update contains 7 significant changes, 5 new monographs and 1 deleted monograph
Significant Changes:
New Monographs:
Deleted Monographs: Hepatitis A with typhoid vaccine.
9th September 2025
This update contains 7 significant changes and 2 dose changes…
9th September 2025
This update contains 7 significant changes and 2 dose changes
Significant Changes:
Dose Changes:
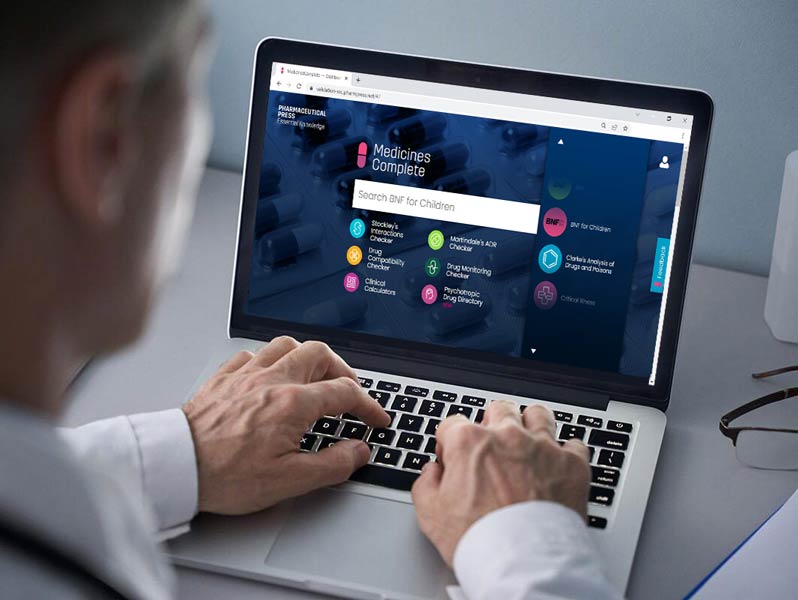
Access BNFC through MedicinesComplete today.
Contact us now for pricing and access information.
BNF and BNFC are published jointly by BMJ Group, Pharmaceutical Press, RCPCH, and NPPG.
A significant investment from National Institute for Health and Care Excellence (NICE) ensures that, with our partners BMJ Group, RCPCH, and NPPG, we support NHS health professionals in the UK with unlimited access to BNF and BNFC content online through MedicinesComplete and via the BNF + BNFC app.

Join us as we celebrate 20 years of BNF for Children (BNFC) supporting health professionals make confident decisions about the safe and effective use of medicines in children.


On-demand Webinar
25th September 2025