10th February 2026
Agilio: Diagnosis and Treatment Guidance
This update contains 6 significant changes and 12 minor changes….


Psychotropic Drug Directory supports the optimal and rational use of medicines, to improve the quality of life for people with mental health needs.
Close
Close

Contact our team to see how our services can directly help you.
Close

For new and experienced users, get the most out of your access to MedicinesComplete with our handy tips and shortcuts. Save time and get to the information you need quickly.
Close

Register for this webinar to learn how MedicinesComplete can support you in implementing effective processes to reduce medication errors and improve patient safety.
Close

Here’s a handy reminder of all the patient safety topics we’ve covered.
Close
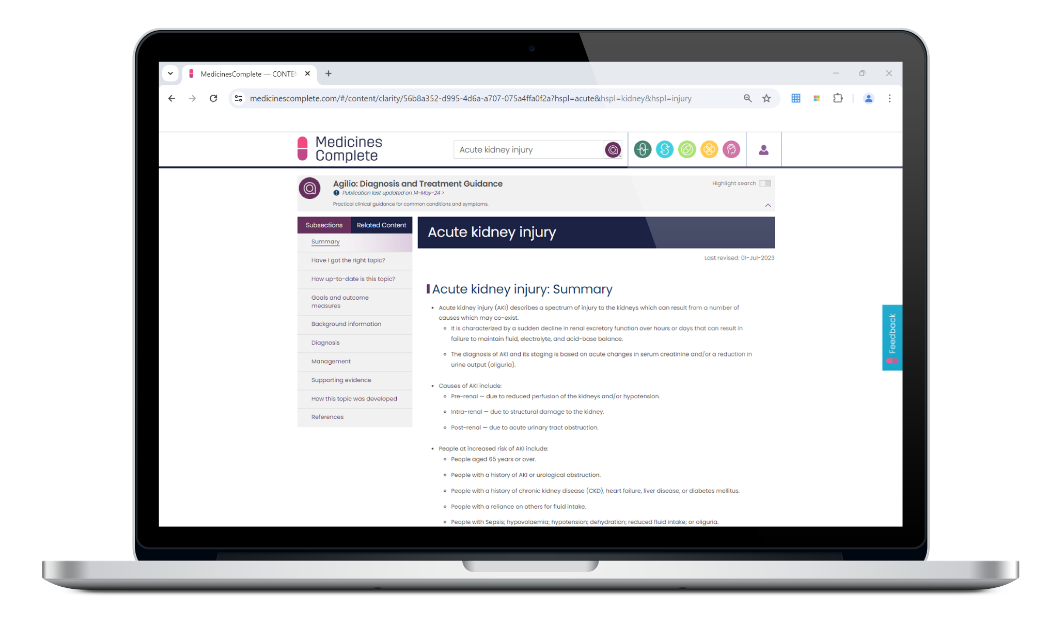

Covering over 370 clinical topics for use in primary care, Agilio: Diagnosis and Treatment Guidance supports confident decision-making at the point of care. Available to customers outside of the UK.
Providing trusted, concise summaries of current evidence and prescribing information to support clinical decisions on a patient’s diagnosis and treatment.
Referenced and linked to source evidence and covering over 1,200 clinical presentations or patient scenarios, this guidance supports health professionals to assess symptoms, diagnose the disease and identify treatment options.








Our knowledge products are regularly updated online through MedicinesComplete
10th February 2026
This update contains 6 significant changes and 12 minor changes….
10th February 2026
This update contains 6 significant changes and 12 minor changes.
Significant Changes:
Minor Changes:
9th December 2025
This update contains 4 significant changes and 8 minor changes….
9th December 2025
This update contains 4 significant changes and 8 minor changes.
Significant Changes:
Minor Changes:
11th November 2025
This update contains 8 significant changes and 11 minor changes….
11th November 2025
This update contains 8 significant changes and 11 minor changes.
Significant changes:
Minor changes:
14th October 2025
This update contains 7 significant changes and 13 minor changes….
14th October 2025
This update contains 7 significant changes and 13 minor changes.
Significant changes:
Minor changes:
9th September 2025
This update contains 12 significant changes and 10 minor changes.Significant…
9th September 2025
This update contains 12 significant changes and 10 minor changes.Significant changes:
Minor changes:
12th August 2025
This update contains 8 significant changes and 11 minor changes….
12th August 2025
This update contains 8 significant changes and 11 minor changes.
Significant Changes:
Minor Changes:
Access Agilio: Diagnosis and Treatment Guidance through MedicinesComplete today.
Contact us now for pricing and access information.


Upcoming Webinar
26th March 2026 – 14:00 GMT (14:00 UTC, 09:00 EST)

Info

Info

Info
See all our printed publications in the Shop.