Our organisation and history
BNF Publications, BNF and BNF for Children, are published jointly by BMJ and Pharmaceutical Press, the publishing division of Royal Pharmaceutical Society.
BNF Publications are independent products that do not market medicines.
Our publications evaluate clinical evidence from diverse sources with information validated by a network of clinical experts and published under the authority of formulary committees. BNF Publications content databases are used to publish all versions of BNF, including our books, apps, and the online version of our publications on MedicinesComplete.
BNF Publications reflect current best practice as well as legal and professional guidelines relating to the uses of medicines. Content includes:
- Guidance on the drug management of common conditions.
- Details of medicines with special reference to their uses, cautions, contra-indications, side-effects, doses, and relative costs.
- Guidance on prescribing, monitoring, dispensing, and administering medicines.
BNF is a direct descendant of the National War Formulary, in which the titles of the preparations were in Latin and the doses in minims and grains. In 1939, the minister for health appointed a committee to prepare a wartime formulary to contain a “selection of medicaments sufficient in range to meet ordinary requirements of therapeutics”. When the war ended, it was the Royal Pharmaceutical Society and the British Medical Association that wanted to continue publication of a formulary for general use. Therefore, BNF was born.
The first edition was published in 1949; an overhauled version of the formulary launched in 1981. Since then, a new book has been published every 6 months, with the cover colour changing for each edition.
The first edition of BNF for Children was published in September 2005 and since then a new book has been published annually.
Today, BNF products are used by health professionals in the UK and abroad, and it has been translated into several languages including Italian, Spanish, Turkish and Polish.
How is BNF funded?
BNF is entirely funded from sales made by the joint publishers, BMJ and Pharmaceutical Press. The publishers sell the publication in both printed and electronic formats in the UK and the rest of the world. Electronic formats are procured by the National Institute for Health and Care Excellence (NICE) for use within NHS organisations in England. Electronic formats are also procured for use within NHS organisations in Scotland and Wales. Printed and electronic formats are procured for use within NHS organisations in Northern Ireland.
Stakeholders
Clinical governance committees
BNF is produced under the authority of a Joint Formulary Committee (JFC), which is responsible for overseeing the content of BNF. BNF for Children is published under the authority of a Paediatric Formulary Committee (PFC), which is responsible for overseeing the content of BNF for Children. Both committees comprise pharmacy, medical, nursing, and lay representatives, as well as representation from UK health departments, national guideline providers, and the Medicines and Healthcare products Regulatory Agency (MHRA), as defined in the standing orders from these committees.
This membership assures a diverse group of stakeholders is involved in the content creation, both from a professional viewpoint (by the range of health professionals involved) and from a wider healthcare context (by including different authorities involved in the use of medicines in the UK). Patients are also represented by the lay members on the committee.
A Nurse Prescribers’ Advisory Group (NPAG) oversees the Nurse Prescribers’ Formulary, a subset of BNF content for Community Practitioner Nurse Prescribers. NPAG includes representatives from organisations such as the Royal College of Nursing, the Community Practitioners’ and Health Visitors’ Association, the Nursing and Midwifery Council, and UK health departments, in addition to practising nurse prescribers from different specialties.
A Dental Advisory Group (DAG) that includes representatives of the British Dental Association and UK health departments, and practising dentists from different specialities, oversees the preparation of advice in BNF Publications on the drug management of dental and oral conditions, and also advises UK health ministers on the list of preparations (found in BNF Publications) that may be prescribed by dental practitioners.
Members of the JFC, NPAG, and DAG are listed within BNF. Members of the PFC, NPAG, and DAG are listed in BNF for Children.
Expert advice
BNF Publications call on representatives from expert groups (such as royal colleges and specialist societies) to advise on the content of the publications. Expert advisers (including doctors, pharmacists, nurses, and dentists) are consulted for independent advice in their area of expertise. These individuals and groups are listed in the acknowledgements section in BNF and BNF for Children.
Feedback
BNF Publications receive much correspondence from practice-based users, which is fed back into content review.
Patient views
Patients’ views are incorporated directly and indirectly into BNF Publications content. The vast majority of BNF Publications content is authored for use by health professionals and, for this content, direct input is via lay representation on the JFC and PFC, and through open peer review of content. In addition, BNF Publications evaluate and incorporate national and international guidance, much of which takes into account patient and user representative’s views in its development. As such, BNF Publications content also indirectly reflect patient views from the perspective of the source material. The only BNF Publications content directly aimed at patients is the Cautionary and Advisory warnings that appear on labels of medicines dispensed to patients. All of these labels were user-tested by patients during development.
Conflicts of interest
All members of clinical governance committees, expert advisers, and those undertaking peer review (described below) are required to declare any potential conflicts of interest; if declared, conflicts are managed appropriately.
Content creation process
BNF Publications follow a rigorous editorial process, to ensure information is correct, up-to-date, and reflects current best practice. Our editorial team come from a variety of practice-based backgrounds and have a sound understanding of how drugs are used in clinical practice.
BNF Publications processes are accredited by the ISO9001:2015 Quality Management System, which covers the management, validation, and processing of medicines information to produce BNF products and services through a range of delivery channels to customers.
NICE started its Accreditation Programme in 2009 to raise standards in guidance production and improve the quality of information produced for health and social care decision-makers; this Programme was closed in 2024. The processes used to create BNF and BNFC content were accredited by NICE in 2016, and accreditation was renewed in 2021. These processes are displayed in the pathway below. Underpinning each stage in the content creation pathway are documented policies, to ensure consistency and rigour at every stage.
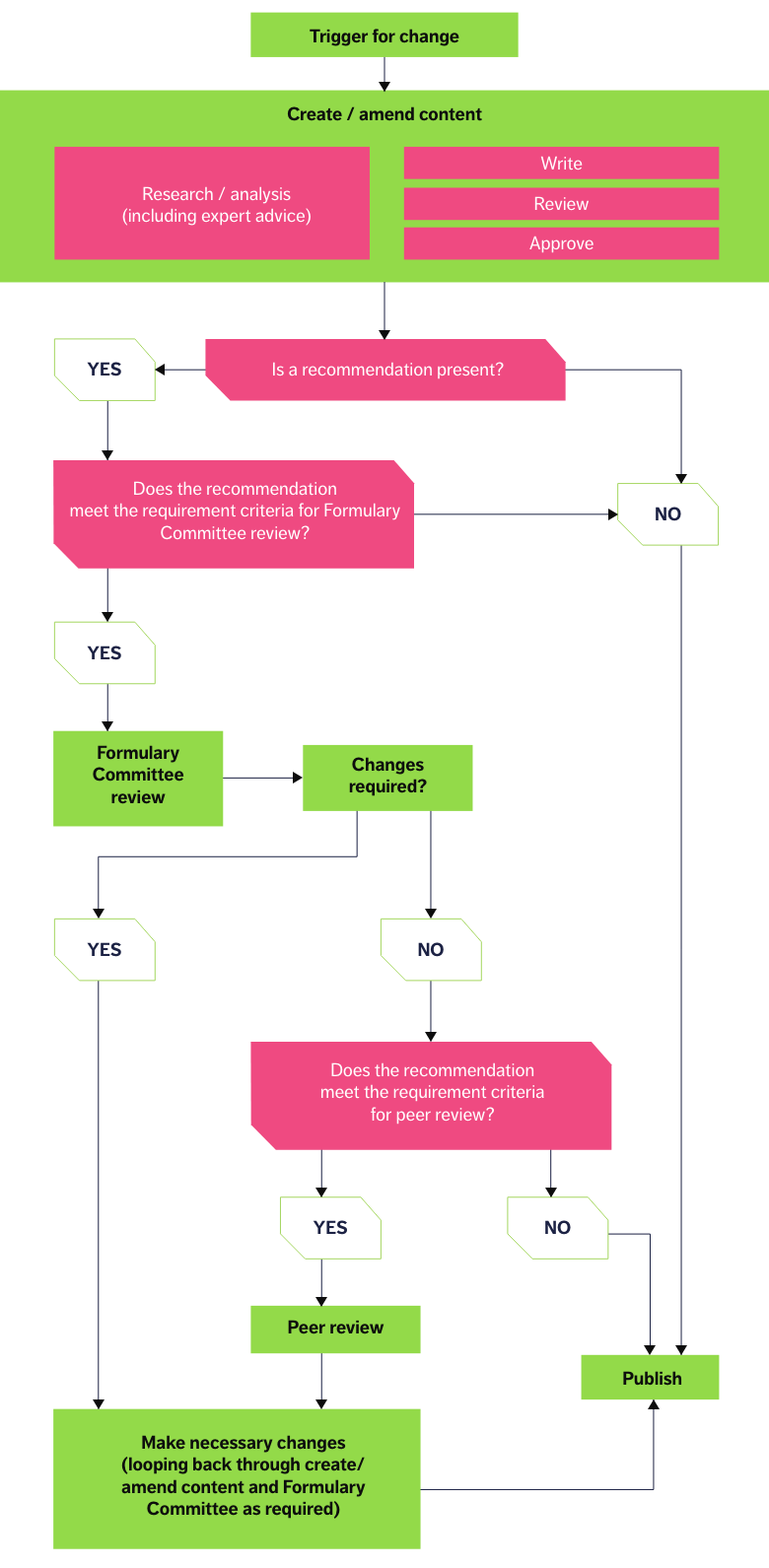
Content types and sources of information
All content within BNF Publications is created using the process pathway described above. However, as depicted, not all content needs to go through every stage — the point in the pathway that content can be published depends on the type of content. BNF Publications content and their typical sources can broadly be classed into four types, as described below:
- Non-clinical facts — categorical information. For example, pack size and price data for medicinal preparations and medical devices found at the end of drug monographs. This content is derived from recognised authority sources, such as the Dictionary of Medicines and Devices (dm+d) and the Drug Tariff, and therefore requires no literature searching in its creation. The source content is updated weekly to monthly; BNF proactively looks for updates in response to this schedule, and updates the content monthly, in line with its online publication cycle.
- Clinical facts — clinical content that is a fact or statement (e.g. a side-effect in monographs or facts about disease states in treatment summaries). The starting point is usually the Summary of Product Characteristics (SPC) or other licensed product information and therefore this initially requires no specific literature searching strategy. However, the content of the SPC may be verified and/or expanded against a number of standard sources. For example, confirming with the World Health Organisation’s International Nonproprietary Names (WHO INN) database that the drug name provided in the SPC is a recommended INN (rINN).
- BNF-summarised recommendation — a recommendation (information that calls a user to action) typically derived from SPCs, published high-quality guidance, or advice from professional bodies or regulatory authorities which are considered as authoritative sources, where the input of BNF is simply to summarise these recommendations.
- BNF-authored recommendation — a recommendation (information that calls a user to action) created by BNF because recommendations from published high-quality guidance or advice from authoritative sources are unavailable or unclear.
For BNF recommendations, the starting point for research is usually a clinical question. The question will initially be addressed by establishing if an SPC is available, and/or high-quality guidelines or advice from authoritative sources that directly answer the clinical question have been published. This may be supplemented by reference to the primary literature. If the clinical question posed can be answered by published literature, the search is complete and a BNF-summarised recommendation is created. Policy documents detail the searches that are to be undertaken, covering the identification of best evidence, the details of the search strategies employed, and the databases used.
BNF Publications use adapted versions of the Appraisal of Guidelines for Research and Evaluation (AGREE II) tool to assess guidelines and the Scottish Intercollegiate Guidelines Network (SIGN) methodology tools to assess other source types. These tools ensure a systematic approach to the critical appraisal of different source types, by checking against standard criteria.
Each type of source (e.g. published guideline, randomised controlled trial, case-control study, expert advice, etc) has a standard template which is used to assess the validity and quality of the source in question. Each template is accompanied by guidance notes intended to further decrease variation in the process.
If the clinical question posed still cannot be answered after literature searching, or if literature searching only returns low-quality evidence, then expert opinion may be sought to create a BNF-authored recommendation. This ensures that expert opinion is only used if high-quality published sources cannot be identified to resolve the clinical question.
Creating and amending content
Once the research phase is complete and a decision has been made to create or amend a recommendation, a minimum three-step internal review process is undertaken to enact such changes. Content is written by a clinical writer, then passed to an independent clinical writer who has not been involved with developing the content to be reviewed (at this stage the content, search strategy, and sources used are reviewed). If rework is required, this process is repeated until the reviewer is in agreement with the drafted content and is satisfied that the required processes and procedures have been followed, then the content goes to a further independent member of the BNF editorial team to approve. Only certain clinical members of the editorial team, usually those with greater experience or specialist knowledge, are permitted to approve content. If rework is required, this process is repeated until the approver is satisfied that the content meets the required standard and is fit for publication.
Formulary committee review
BNF Publications content creation is overseen by the formulary committees (described above). Once the internal team and expert advisers (if applicable) are satisfied that the content created is fit for publication, the JFC/PFC performs additional review of more complex or contentious pieces of content to ensure that the intention of the source literature has not been misinterpreted, and that the content is fit for publication.
Peer review
Recommendations will only be sent for peer review when they have been through all of the content creation steps, and agreed to in principle by the relevant formulary committee, but created significant debate within the committee and/or were subject to a chair’s decision. The editorial team also reserves the right to send recommendations for peer review if there is felt to be particular additional benefit. While the peer review process helps assure the quality, validity, or relevance of the recommendation to the user base, it can also delay publication of information. The benefit of quick publication is deemed to outweigh the benefits of peer review when the recommendation being published has been derived from a source that is produced according to a robust methodology which in itself has been peer reviewed, and therefore further peer review offers minimal additional advantage. BNF Publications therefore carefully select which information is submitted to peer review in order to achieve the best balance between the quality assurance aspects peer review delivers and the stated aims of BNF Publications.
Ongoing review
BNF Publications ensure that once published, all clinical recommendations are reviewed and updated in the light of new evidence if necessary. Reviews are carried out both proactively and reactively, with the aim of considering all recommendations for review every 3 to 4 years.
Clarity and presentation
As BNF Publications provide concise information, it is not practical for all of the sources used to be referenced throughout the text. Furthermore, in a national consultation, full referencing was identified as undesirable by our end users.
As such, clinical recommendations in BNF Publications are managed in the following ways:
- Links to key guidelines used in the creation of recommendations are provided under the heading ‘Useful resources’ within treatment summaries (e.g. Hyperparathyroidism treatment summary).
- The source of a recommendation or statement (but not the full reference) may be made explicit in the text allowing the authority behind the recommendation or statement to be clearly identified; a link to the source material may also be provided (e.g. safety statements derived from the MHRA’s Drug Safety Updates are identified as such in the relevant content).
- For other clinical recommendations, the user is shown the strength of the recommendation being made by a simple A to E evidence grading system (see below). Recommendations from SPCs may either be preceded by “manufacturer advises” or have the symbol “M” (manufacturer information) displayed next to the recommendation within the text.
Evidence grading system
BNF Publications have adopted a five-level grading system from A (highest) to E (lowest), adapted from the former SIGN grading system. This grade is displayed next to the recommendation within the text.
Evidence used to make a recommendation is assessed for validity using standardised methodology tools based on AGREE II and assigned a level of evidence. The recommendation is then given a grade that is extrapolated from the level of evidence, and an assessment of the body of evidence and its applicability.
Evidence assigned a level 1- or 2- score has an unacceptable level of bias or confounding and is not used to form recommendations.
Levels of evidence
Level 1++
High-quality meta-analyses, systematic reviews of randomised controlled trials (RCTs), or RCTs with a very low risk of bias.
Level 1+
Well-conducted meta-analyses, systematic reviews, or RCTs with a low risk of bias.
Level 1-
Meta-analyses, systematic reviews, or RCTs with a high risk of bias.
Level 2++
High-quality systematic reviews of case control or cohort studies; or high-quality case control or cohort studies with a very low risk of confounding or bias and a high probability that the relationship is causal.
Level 2+
Well-conducted case control or cohort studies with a low risk of confounding or bias and a moderate probability that the relationship is causal.
Level 2-
Case control or cohort studies with a high risk of confounding or bias and a significant risk that the relationship is not causal.
Level 3
Non-analytic studies, e.g. case reports, case series.
Level 4
Expert advice or clinical experience from respected authorities.
Grades of recommendation
Grade A: High strength
NICE-accredited guidelines; or other guidelines, assessed using AGREE II, that meet the grade A threshold; or at least one meta-analysis, systematic review, or RCT rated as 1++, and directly applicable to the target population; or a body of evidence consisting principally of studies rated as 1+, directly applicable to the target population, and demonstrating overall consistency of results.
Grade B: Moderate strength
Guidelines, assessed using AGREE II, that meet the grade B threshold; or a body of evidence including studies rated as 2++, directly applicable to the target population, and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 1++ or 1+.
Grade C: Low strength
Guidelines, assessed using AGREE II, that meet the grade C threshold; or a body of evidence including studies rated as 2+, directly applicable to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2++.
Grade D: Very low strength
Guidelines, assessed using AGREE II, that meet the grade D threshold; or evidence level 3; or extrapolated evidence from studies rated as 2+; or tertiary reference source created by a transparent, defined methodology, where the basis for recommendation is clear.
Grade E: Practice point
Evidence level 4.
Sign up to the BNF monthly newsletter
Sign up to keep up to date with the latest new drugs and significant changes to BNF and BNF for Children.

Create your own customised formulary
Powered by BNF, FormularyComplete allows you to create your own customised formulary, supporting you locally to make quick, clinically informed decisions.
Access BNF content online, on the move and in print
BNF and BNF for Children are available online through MedicinesComplete.
The essential app for iOS and Android to access medicines information on-the-go.

Essential reference books published in updated book form twice a year.

BNF content is available for licensing through Pharmaceutical Press.









