 Extended Stability for Parenteral Drugs
Extended Stability for Parenteral Drugs
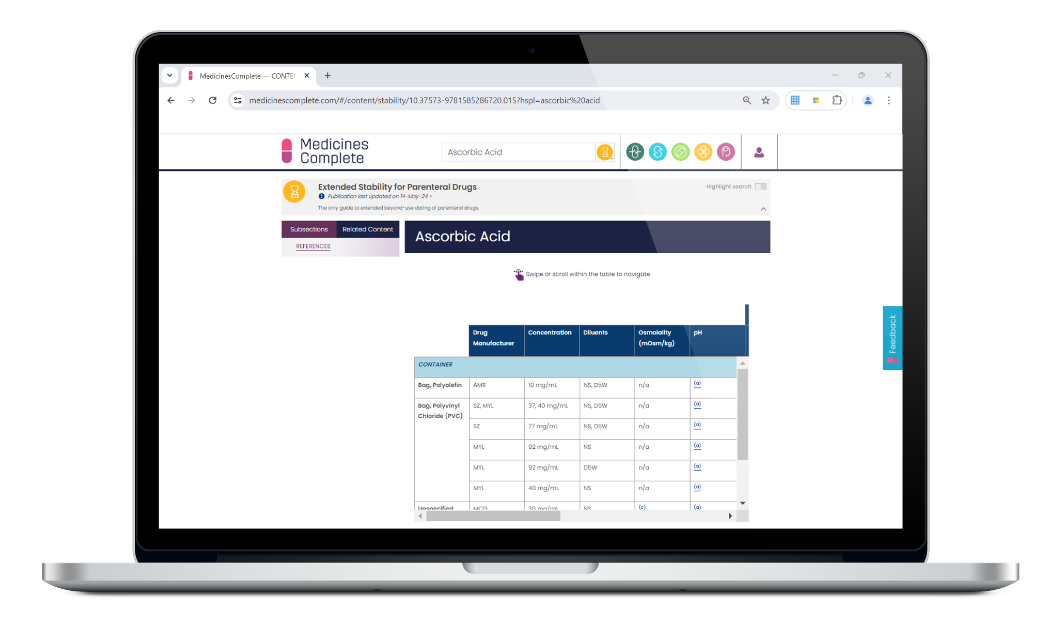

The only guide to safely extend the dating of parenteral drugs
Extended Stability for Parenteral Drugs supports safe extended dating of parenteral drugs beyond the usual 24-hour limit – minimising waste and lowering medicines costs. The go-to reference for anyone preparing, administering, or compounding.
Extended Stability for Parenteral Drugs provides an extensive list of medications with temperature excursion data for intact vials. Coverage of all aspects of determining stability, including the changing elastomeric landscape and the ongoing variability in stability data. Plus, newly published data on parenteral nutrition, oncology, specialty, and COVID medications.
- 196
- Monographs
- 37
- New stability monographs
- 808
- References and communication documents
- 2
- Editors with 13 contributing writers
Safely extend beyond-use dating of parenteral medications

Quickly access extended stability information for IV solutions
Safely extend dating of parenteral medications

Minimise medicines waste from partial dosing or temperature excursions

Reduce medicines costs

Allows medicines to be prepared in bulk or ahead of time

Enable optimal patient administration

Links to ASHP Injectable Drug Information

User-friendly data in easy to digest tables
Request access today
Access Extended Stability for Parenteral Drugs through MedicinesComplete today.
Contact us now for pricing and access information.
Related resources


Upcoming Webinar
24th April 2025 – 14:00 BST (13:00 UTC, 09:00 EDT)
From evidence to trusted knowledge: How our expert editorial team create actionable guidance to support confident decisions
Related publications
-

-

Prescribing Medicines for Children From drug development to practical administration
Your guide to delivering safe and effective medicines for children.
Info -
 BEST SELLER
BEST SELLERDrugs in Use: Case Studies for Pharmacists and Prescribers Sixth Edition
Bridges the gap between theoretical knowledge about medicines and its practical application to patie…
Info
See all our printed publications in the Shop.














