11th March 2025
BNF for Children
This update contains 1 significant change and 2 new preparations….


Psychotropic Drug Directory supports the optimal and rational use of medicines, to improve the quality of life for people with mental health needs.
Close
Close

Contact our team to see how our services can directly help you.
Close

For new and experienced users, get the most out of your access to MedicinesComplete with our handy tips and shortcuts. Save time and get to the information you need quickly.
Close

Register for this webinar to learn how MedicinesComplete can support you in implementing effective processes to reduce medication errors and improve patient safety.
Close
Learn how to calculate drug dosage for special populations in our article.
Close

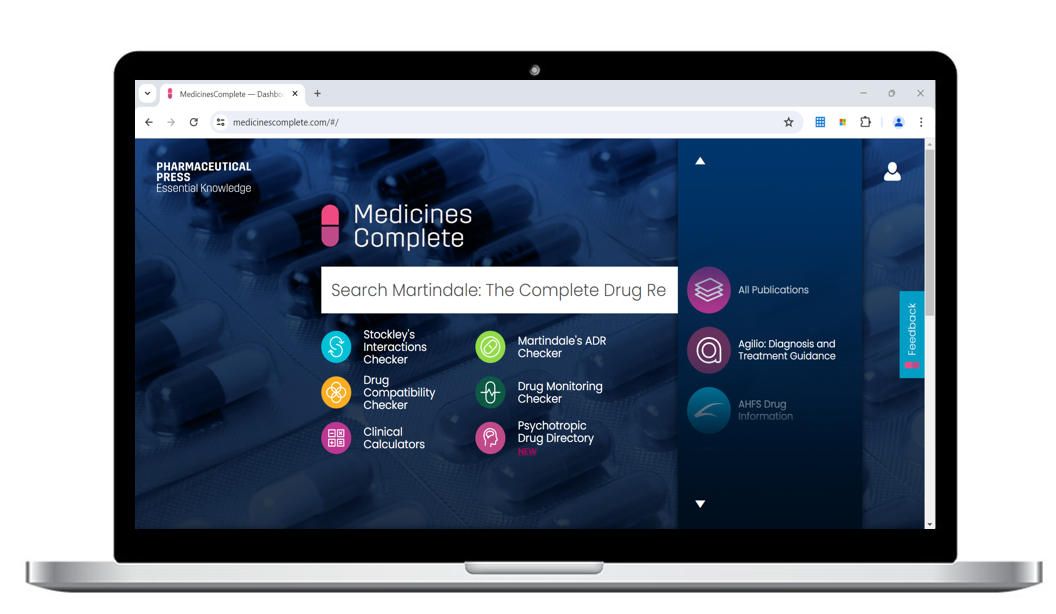

Essential knowledge to help you make the right decision.
MedicinesComplete makes it easy for health and care professionals to access trusted evidence-based guidance to support confident decision making at the point of need.
Use this page for information, training, and guides to help you get the most out of your access to this trusted online platform.

Esbonia’r canllaw defnyddiwr hwn sut i gyrchu a llywio MedicinesComplete, ynghyd â chynnig
canllawiau ar sut i ddefnyddio cyhoeddiadau penodol.
MedicinesComplete brings together trusted knowledge products providing medicines information and expert guidance on the safe use and administration of drugs and medicines, in one place.








Our knowledge products are regularly updated online through MedicinesComplete
11th March 2025
This update contains 1 significant change and 2 new preparations….
11th March 2025
This update contains 1 significant change and 2 new preparations.
Significant Changes:
New Preparations: Hyftor® [sirolimus]; Verorab® [rabies vaccine].
11th March 2025
This update contains 3 significant changes, 3 new monographs and…
11th March 2025
This update contains 3 significant changes, 3 new monographs and 3 new preparations.
Significant Changes:
New Monographs:
New Preparations: Cequa® [ciclosporin]; Hyftor® [sirolimus]; Verorab® [rabies vaccine].
11th March 2025
This update contains 10 new drugs, 29 minor updates, 100…
11th March 2025
This update contains 10 new drugs, 29 minor updates, 100 new or updated pieces of short management advice, and 7 existing drugs have been revalidated.
11th March 2025
This update contains 4 new monographs, 38 revised monographs and…
11th March 2025
This update contains 4 new monographs, 38 revised monographs and 23 discontinued Monographs.
New monographs: Crinecerfont; Landiolol Hydrochloride; Olezarsen Sodium; Remestemcel-L-rknd.
Revised monographs: Abacavir Sulfate; Benzgalantamine Gluconate; Cabotegravir and Rilpivirine; Cabotegravir, Cabotegravir Sodium; Crisaborole; Dapagliflozin Propanediol; Darbepoetin Alfa; Deutetrabenazine; Dolutegravir Sodium and Lamivudine; Doravirine; Doravirine, Lamivudine, and Tenofovir Disoproxil Fumarate; DULoxetine Hydrochloride; Duvelisib; Eculizumab; Emtricitabine; Etravirine; Fezolinetant; Fosamprenavir Calcium; Guselkumab; Iloprost (Frostbite); Lazertinib Mesylate; Lonapegsomatropin-tcgd; Lotilaner; Macitentan and Tadalafil; Mavacamten; Metoclopramide Hydrochloride; Nelfinavir Mesylate; Nirmatrelvir and Ritonavir; Ravulizumab-cwvz; Respiratory Syncytial Virus Vaccine; Respiratory Syncytial Virus Vaccine, Adjuvanted; Ribociclib Succinate; Selpercatinib; Sparsentan; Tipranavir; Velmanase Alfa-tycv; Voclosporin; Zuranolone.
Discontinued monographs: Aducanumab; Antiretroviral General Statement; Bamlanivimab and etesevimab; Bebtelovimab; Belladonna; Casirivimab and imdevimab; Cefotaxime; Ceftobiprole; Copanlisib; HMG-CoA Reductase Inhibitors General Statement; Infigratinib; Insulin detemir; Olaratumab; Omacetaxine; Oprelvekin; Peginterferon alfa-2b; Rosiglitazone; Sodium phenylbutyrate and taurursodiol; Sotrovimab; Tixagevimab and cilgavimab; Vitamins A and D; Vorapaxar; Voxelotor.
11th March 2025
For March, 290 new interactions have been added, and 73…
11th March 2025
For March, 290 new interactions have been added, and 73 existing interactions have been updated.
11th March 2025
This update contains 4 new monographs and 29 existing monographs…
11th March 2025
This update contains 4 new monographs and 29 existing monographs have been updated. Preparations have also been updated for 4 countries.
New Monographs
Selective Updates – some new, re-validated or changed information
Monographs
Updated names, synonyms and codes; official standards; official preparations
Proprietary Preparations and Manufacturers updated for the following countries and regions:
† Countries updated on an ongoing basis
Stay updated on the latest new drugs and significant changes to medicines information on MedicinesComplete when you sign up to our monthly newsletter.

For deeper information about essential knowledge products on MedicinesComplete browse our library of on-demand webinars featuring our expert editorial team, or register for our latest live webinar.

![]()
Facilitates the exchange of information between colleagues on matters relating to drug and symptom control, non-drug and service issues in palliative and hospice care. Register to take part today.

For support, to provide feedback on our content, suggest ideas for new content, or for any other enquiry please get in touch.

If you are interested in creating or contributing to our content on MedicinesComplete please use the link below to find out more.