8th April 2025
AHFS Drug Information
This update contains 9 new monographs and 44 revised monographs….


Psychotropic Drug Directory supports the optimal and rational use of medicines, to improve the quality of life for people with mental health needs.
Close
Close

Contact our team to see how our services can directly help you.
Close

For new and experienced users, get the most out of your access to MedicinesComplete with our handy tips and shortcuts. Save time and get to the information you need quickly.
Close

Register for this webinar to learn how MedicinesComplete can support you in implementing effective processes to reduce medication errors and improve patient safety.
Close

Read about therapeutic drug monitoring in our article.
Close
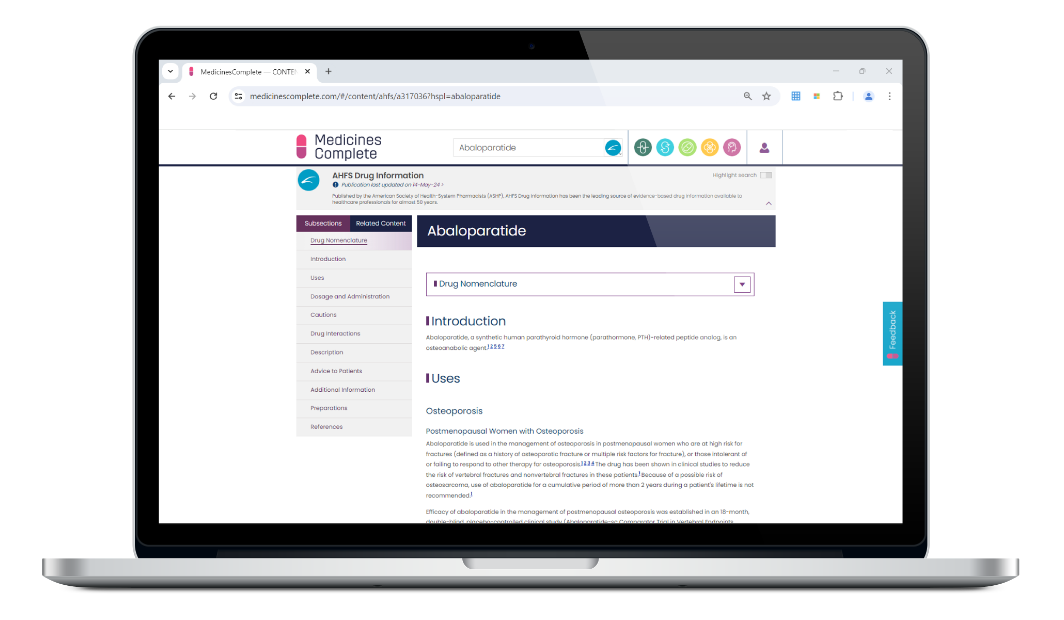

Published by the American Society of Health-System Pharmacists (ASHP), AHFS Drug Information is a dependable, comprehensive, and objective database for drug products approved by the FDA.
The American Hospital Formulary Service (AHFS) drug information database is maintained and updated continuously throughout the year.
The inclusion of evidence-based data on licensed and unlicensed drug use makes this a valuable reference for health professionals seeking to provide their patients with safe and effective therapy.







Our knowledge products are regularly updated online through MedicinesComplete
8th April 2025
This update contains 9 new monographs and 44 revised monographs….
8th April 2025
This update contains 9 new monographs and 44 revised monographs.
New monographs: Concizumab; Cosibelimab; Datopotamab Deruxtecan; Elagolix Sodium, Estradiol, and Norethindrone Acetate; Ensartinib Hydrochloride; Suzetrigine; Treosulfan; Vanzacaftor Calcium, Tezacaftor, and Deutivacaftor; Zenocutuzumab.
Revised monographs: Antihemophilic Factor (Recombinant), Fc-VWF-XTEN Fusion Protein-ehtl; Avacincaptad Pegol Sodium; Belumosudil Mesylate; Belzutifan; Brodalumab; cefTRIAXone Sodium; Certolizumab Pegol; Darunavir Ethanolate, Cobicistat, Emtricitabine, and Tenofovir Alafenamide Fumarate; Dolutegravir Sodium; Dolutegravir Sodium and Rilpivirine Hydrochloride; Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Alafenamide Fumarate; Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate; Emtricitabine and Tenofovir Disoproxil Fumarate; Evolocumab; fentaNYL, fentaNYL Citrate; Fostemsavir Tromethamine; Glatiramer Acetate; Ibalizumab; Ibuprofen, Ibuprofen Lysine; lamiVUDine; Lonafarnib; Lopinavir and Ritonavir; Lurasidone Hydrochloride; Maraviroc; Methotrexate; Mirtazapine; Molnupiravir; Nabumetone; Nemolizumab; Pimavanserin Tartrate; Raltegravir Potassium; Remdesivir; Remifentanil Hydrochloride; Ribavirin; Sparsentan; Sulbactam Sodium and Durlobactam Sodium; Tazemetostat Hydrobromide; Teclistamab; Teduglutide; Tenofovir Alafenamide Fumarate; Tenofovir Disoproxil Fumarate; Toripalimab; Vilobelimab; Zidovudine.
11th March 2025
This update contains 4 new monographs, 38 revised monographs and…
11th March 2025
This update contains 4 new monographs, 38 revised monographs and 23 discontinued Monographs.
New monographs: Crinecerfont; Landiolol Hydrochloride; Olezarsen Sodium; Remestemcel-L-rknd.
Revised monographs: Abacavir Sulfate; Benzgalantamine Gluconate; Cabotegravir and Rilpivirine; Cabotegravir, Cabotegravir Sodium; Crisaborole; Dapagliflozin Propanediol; Darbepoetin Alfa; Deutetrabenazine; Dolutegravir Sodium and Lamivudine; Doravirine; Doravirine, Lamivudine, and Tenofovir Disoproxil Fumarate; DULoxetine Hydrochloride; Duvelisib; Eculizumab; Emtricitabine; Etravirine; Fezolinetant; Fosamprenavir Calcium; Guselkumab; Iloprost (Frostbite); Lazertinib Mesylate; Lonapegsomatropin-tcgd; Lotilaner; Macitentan and Tadalafil; Mavacamten; Metoclopramide Hydrochloride; Nelfinavir Mesylate; Nirmatrelvir and Ritonavir; Ravulizumab-cwvz; Respiratory Syncytial Virus Vaccine; Respiratory Syncytial Virus Vaccine, Adjuvanted; Ribociclib Succinate; Selpercatinib; Sparsentan; Tipranavir; Velmanase Alfa-tycv; Voclosporin; Zuranolone.
Discontinued monographs: Aducanumab; Antiretroviral General Statement; Bamlanivimab and etesevimab; Bebtelovimab; Belladonna; Casirivimab and imdevimab; Cefotaxime; Ceftobiprole; Copanlisib; HMG-CoA Reductase Inhibitors General Statement; Infigratinib; Insulin detemir; Olaratumab; Omacetaxine; Oprelvekin; Peginterferon alfa-2b; Rosiglitazone; Sodium phenylbutyrate and taurursodiol; Sotrovimab; Tixagevimab and cilgavimab; Vitamins A and D; Vorapaxar; Voxelotor.
11th February 2025
This update contains 6 new monographs and 21 revised monographs….
11th February 2025
This update contains 6 new monographs and 21 revised monographs.
New monographs: Acoramidis Hydrochloride; Eladocagene Exuparvovec-tneq; Obecabtagene Autoleucel; Revumenib Citrate; Sulopenem etzadroxil and probenecid; Zanidatamab-hrii.
Revised monographs: Alpelisib; Aprocitentan; Aztreonam; Copyright and Notices; Empagliflozin; Ensifentrine; Epcoritamab-bysp; Furosemide; Iptacopan Hydrochloride; Ivacaftor; Ketamine Hydrochloride; Losartan Potassium; Obeticholic Acid; Omalizumab; Osimertinib Mesylate; Respiratory Syncytial Virus Vaccine (mRNA); Respiratory Syncytial Virus Vaccine; Respiratory Syncytial Virus Vaccine, Adjuvanted; Risankizumab-rzaa; Ustekinumab; Vericiguat.
14th January 2025
This update contains 4 new monographs and 13 revised monographs….
14th January 2025
This update contains 4 new monographs and 13 revised monographs.
New monographs: Foscarbidopa and Foslevodopa; Inavolisib; Marstacimab-hncq;
Zolbetuximab-clzb.
Revised monographs: Aminocaproic Acid; Apalutamide; Apremilast; Cefiderocol; Celecoxib; Coagulation Factor Xa (Recombinant), Inactivated-zhzo (Systemic); Influenza Vaccine Inactivated; Lisinopril; Ponatinib; Ripretinib; Sarilumab; Tenapanor; Tocilizumab.
10th December 2024
This update contains 8 new monographs and 39 revised monographs….
10th December 2024
This update contains 8 new monographs and 39 revised monographs.
New monographs: Arimoclomol Citrate; Artesunate; Azithromycin (EENT); Denileukin diftitox-cxdl; Deuruxolitinib Phosphate; Lebrikizumab-lbkz; Levacetylleucine; Xanomeline Tartrate and Trospium Chloride.
Revised monographs: Adagrasib; Adalimumab; Alectinib Hydrochloride; Benralizumab; Casimersen; Danicopan; Deflazacort; Dronabinol; Dupilumab; Edaravone; Eflornithine Hydrochloride; Enfuvirtide; Estrogen-Progestin Combinations; Etrasimod Arginine; Ixazomib Citrate; Ixekizumab; Lanreotide Acetate; Leniolisib Phosphate; Lidocaine Hydrochloride (Systemic); Lomustine; Midazolam, Midazolam Hydrochloride; Mirikizumab-mrkz; Nedosiran Sodium; Ozanimod Hydrochloride; Pirtobrutinib; Pozelimab-bbfg; Repotrectinib; Resmetirom; Riluzole; Sotatercept-csrk; Tepotinib Hydrochloride; Tisotumab vedotin-tftv; Tofersen; Tovorafenib; Tralokinumab-ldrm; Vadadustat; Vasopressin; Vedolizumab; Zanubrutinib.
12th November 2024
This update contains 9 new monographs and 38 revised monographs….
12th November 2024
This update contains 9 new monographs and 38 revised monographs.
New monographs: Axatilimab-csfr; Benzgalantamine Gluconate; Lazertinib Mesylate; Levamlodipine Maleate; Nemolizumab-ilto; Omidubicel-onlv; Palopegteriparatide; Seladelpar Lysine; Vorasidenib.
Revised monographs: Alpelisib; Alteplase; Atorvastatin Calcium; Belimumab; Bictegravir Sodium, Emtricitabine, and Tenofovir Alafenamide Fumarate; Brexanolone; Brincidofovir; Capecitabine; Capivasertib; Cidofovir; COVID-19 Vaccine, Adjuvanted (Novavax)(2024-2025 Formula); COVID-19 Vaccine, mRNA (Moderna) (2024-2025 Formula); COVID-19 Vaccine, mRNA (Pfizer-BioNTech); DULoxetine Hydrochloride; Efavirenz; Emtricitabine and Tenofovir Alafenamide Fumarate; Eplontersen Sodium; Influenza Vaccine Live Intranasal; Influenza Vaccine Recombinant; Insulin Human; Ponesimod; Remifentanil Hydrochloride; Ritonavir; Rosuvastatin Calcium; Simvastatin; Siponimod Fumarate; Smallpox (Vaccinia) Vaccine Live; Smallpox and Mpox Vaccine Live; Talazoparib Tosylate; Teclistamab-cqyv; Tecovirimat; Temozolomide; Tenapanor Hydrochloride (Phosphate-Removing Agent); Teriflunomide; Vaccinia Immune Globulin IV (Human); Vamorolone; Vandetanib; Zuranolone.
Access AHFS Drug Information through MedicinesComplete today.
Contact us now for pricing and access information.


Upcoming Webinar
24th April 2025 – 14:00 BST (13:00 UTC, 09:00 EDT)

Info

Info

Info
See all our printed publications in the Shop.